CHEM Research Interests
We direct our research interests into two groups, the Bio and Condensed Phase Chemistry group, a joint group with the Biology department and led by Roberto Alonso Mori, and the Gas Phase Photochemistry group, a joint group with the AMOS department and led by Ming-Fu Lin.
The gas phase photochemistry group is lead by Ming-Fu Lin and interested in ultrafast photochemical dynamics triggered by valence photoexcitation. Some group members are affiliated with the "Excited States in Isolated Molecules" thrust of the Stanford PULSE Institute and the group closely collaborates with PULSE scientists on its research. The group performs research using the unique capabilities of LCLS and develops LCLS instrumentation to enable transformative research in the field of gas phase photochemistry. Below are details on three active R&D areas the gas phase photochemistry group is involved in:

Investigation of Structural Dynamics Using Ultrafast Diffraction Methods

The group has been closely involved in the development of the gas phase experimental endstation of the megaelectronvolt ultrafast electron diffraction (MeV-UED) instrument.[1] A special focus of the group's R&D work is the development of sample sources to widen the science case of gas phase structural dynamics research at MeV-UED. Examples are the highly successful flow cell design for high vapor pressure samples with compatibility to high repetition reates and the ongoing development of a slit jet design for low vapor pressure sample molecules. The group also supports gas phase X-ray diffraction experiments at the CXI hutch.
These instrument development efforts tie closely into the research interests of the group.[2] They range from investigating the details of electrocyclic reactions[3,4] to carbon-halogen bond dissociation dynamics,[5] and observing the correlated motion of electrons and nuclei during nonadiabatic dynamics.[6]
Publications
[1] X. Shen, J. Pedro F. Nunes, J. Yang, K. Jobe, R. Li, M.-F. Lin, B. Moore, M. Niebuhr, S. Weathersby, T. J. A. Wolf, C. Yoneda, M. Gühr, M. Centurion, X. Wang, Femtosecond gas-phase mega-electron-volt ultrafast electron diffraction, Struct. Dyn., 6, 054305 (2019).
[2] M. Centurion, T. J. A. Wolf, J. Yang, Ultrafast Imaging of Molecules with Electron Diffraction, Annu. Rev. Phys. Chem., DOI: 10.1146/annurev-physchem-082720-010539
[3] T. J. A. Wolf, D. M. Sanchez, J. Yang, R. M. Parrish, J. P. F. Nunes, M. Centurion, R. Coffee, J. P. Cryan, M. Gühr, K. Hegazy, A. Kirrander, R. K. Li, J. Ruddock, X. Shen, T. Veccione, S. P. Weathersby, P. M. Weber, K. Wilkin, H. Yong, Q. Zheng, X. J. Wang, M. P. Minitti, T. J. Martínez, The photochemical ring-opening of 1,3-cyclohexadiene imaged by ultrafast electron diffraction, Nat. Chem. 11, 504 – 509, (2019).
[4] E. G. Champenois, D. M. Sanchez, J. Yang, J. P. F. Nunes, A. Attar, M. Centurion, R. Forbes, M. Gühr, K. Hegazy, F. Ji, S. K. Saha, Y. Liu, M.-F. Lin, D. Luo, B. Moore, X. Shen, M. R. Ware, X. J. Wang, T. J. Martínez, T. J. A. Wolf, Conformer-specific Photochemistry Imaged in Real Space and Time, Science, 374, 178 (2021).
[5] J. Yang, X. Zhu, T. J. A. Wolf, Z. Li, J. P. F. Nunes, R. Coffee, J. Cryan, M. Gühr, K. Hegazy, T. F. Heinz, K. Jobe, R. Li, X. Shen, T. Veccione, S. Weathersby, K. J. Wilkin, C. Yoneda, Q. Zheng, T. J. Martinez, M. Centurion, X. Wang, Imaging CF3I conical intersection and photodissociation dynamics by ultrafast electron diffraction, Science, 361, 64 (2018).
[6] J. Yang, X. Zhu, J. P. F. Nunes, J. K. Yu, R. M. Parrish, T. J. A. Wolf, M. Centurion, M. Gühr, R. Li, Y. Liu, B. Moore, M. Niebuhr, S. Park, X. Shen, S. Weathersby, T. Weinacht, T. J. Martinez, X. Wang, Simultaneous observation of nuclear and electronic dynamics by ultrafast electron diffraction, Science, 368, 885 (2020).
Tracking Photochemical Dynamics with Time-resolved Soft X-ray Spectroscopy
The group actively performs and supports photochemistry research using soft X-ray observables at the TMO instrument.[1-3] Members of the group demonstrated the use of time-resolve soft X-ray absorption spectroscopy to follow excited state population dynamics with sensitivity to the electronic state character.[4] It is involved in the R&D efforts around the high repetition rate LCLS-II upgrade, which will enable novel and transformative experimental methods based on coincidence techniques.
Publications:
[1] T. J. A. Wolf, A. C. Paul, S. D. Folkestad, R. H. Myhre, J. P. Cryan, N. Berrah, P. H. Bucksbaum, S. Coriani, G. Coslovich, R. Feifel, T. J. Martinez, S. P. Moeller, M. Mucke, R. Obaid, O. Plekan, R. J. Squibb, H. Koch, M. Gühr, Transient Resonant Auger-Meitner Spectra of Photoexcited Thymine, Faraday Discuss., 228, 555 (2021).
[2] T. J. A. Wolf, M. Gühr, Photochemical Pathways in Nucleobases measured with an X-ray FEL, Philos. Trans. Royal Soc. A, 377, 20170473 (2019).
[3] L. Inhester, Z. Li, X. Zhu, N. Medvedev, T. J. A. Wolf, Characterization of chemical bond dissociation using femtosecond core-level electron spectroscopy, J. Phys. Chem. Lett., 10, 6536 (2019).
[4] T. J. A. Wolf, R. H. Myhre, J. P. Cryan, S. Coriani, R. J. Squibb, A. Battistoni, N. Berrah, C. Bostedt, P. Bucksbaum, G. Coslovich, R. Feifel, K. J. Gaffney, J. Grilj, T. J. Martínez, S. Miyabe, S. P. Moeller, M. Mucke, A. Natan, R. Obaid, T. Osipov, O. Plekan, S. Wang, H. Koch, M. Gühr, Probing ultrafast ππ* / nπ* internal conversion in organic chromophores via K-edge resonant absorption, Nat. Commun. 8, 29 (2017).
Liquid Phase Ultrafast Electron Diffraction
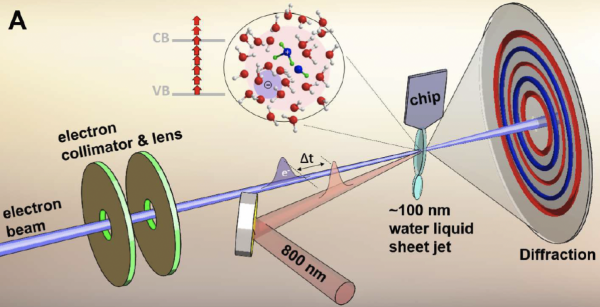
The group collaborated with scientists from PULSE, the accelerator department, the sample environment department at LCLS, and the University of Nebraska, Lincoln, to design a liquid phase endstation, which enabled the first liquid phase electron diffraction experiments in free-standing jets.[1,2] The project lead to seminal studies of hydrogen bonding dynamics[3] and the hydroxyl-hydronium pair[4] in liquid water and the dissocation dynamics of the I3- ion in water solution.[5]
Publications:
[1] J. Yang, J. P. F. Nunes, K. Ledbetter, E. Biasin, M. Centurion, Z. Chen, A. A. Cordones, C. Crissman, D. P. Deponte, S. H. Glenzer, M.-F. Lin, M. Mo, C. D. Rankine, X. Shen, T. J. A. Wolf, X. Wang, Structure retrieval in liquid-phase electron scattering, Phys. Chem. Chem. Phys., 23, 1308 (2021).
[2] J. P. F. Nunes, K. Ledbetter, M. Lin, M. Kozina, D. P. DePonte, E. Biasin, M. Centurion, C. J. Crissman, M. Dunning, S. Guillet, K. Jobe, Y. Liu, M. Mo, X. Shen, R. Sublett, S. Weathersby, C. Yoneda, T. J. A. Wolf, J. Yang, A. A. Cordones, X. J. Wang, Liquid-phase Mega-electron-volt ultrafast electron diffraction, Struct. Dyn., 7, 024301 (2020).
[3] J. Yang, R. Dettori, J. P. F. Nunes, N. H. List, E. Biasin, M. Centurion, Z. Chen, A. A. Cordones, D. P. Deponte, T. F. Heinz, M. E. Kozina, K. Ledbetter, M.-F. Lin, A. M. Lindenberg, M. Mo, A. Nilsson, X. Shen, T. J. A. Wolf, D. Donadio, K. J. Gaffney, T. J. Martinez, X. Wang, Direct Observation of Ultrafast Hydrogen Bond Strengthening in Liquid Water, Nature, 596, 531 (2021).
[4] M.-F. Lin, N. Singh, S. Liang,M. Mo, J. P. F. Nunes, K. Ledbetter, J. Yang, M. Kozina, S. Weathersby, X. Shen, A. A. Cordones, T. J. A. Wolf, C. D. Pemmaraju, M. Ihme, X. J. Wang, Imaging the short-lived hydroxyl-hydronium pair in ionized liquid water, Science, 374, 92-95 (2021).
[5] K. Ledbetter, E. Biasin, J. P. F. Nunes, M. Centurion, K. J. Gaffney, M. Kozina, M.-F. Lin, X. Shen, J. Yang, X. J. Wang, T. J. A. Wolf, A. A. Cordones, Photodissociation of aqueous I3- observed with liquid-phase ultrafast mega-electron-volt electron diffraction, Struct. Dyn., 7, 064901 (2020).
Tracking complex photochemical dynamics with novel spectroscopy methods enabled by LCLS-II
LCLS is currently undergoing an upgrade to increase its repetition rate from 120 Hz up to 1 MHz. The increase of repetition rate and average photon flux will enable transformative experiments using e.g. coincidence techniques at the DREAM endstation, which is currently being assembled. We are preparing to exploit the new experimental opportunities to study complex photochemical reaction landscapes which are difficult to investigate with ensemble-based methods.

The Bio and Condensed Phase Chemistry (BCPC) group is led by Roberto Alonso-Mori and interested in ultrafast photochemical dynamics in condensed phase. The group performs research using the unique capabilities of LCLS and develops instrumentation to enable transformative research in the field of condensed phase photochemistry and biochemistry. The group, joint between the LCLS Chemical and Biology Sciences Departments, is composed of people with a wide variety of scientific interests and drives a portfolio of R&D projects. The main activities of the group are described below.
Condensed Phase Ultrafast Photochemistry, Excited-state chemical dynamics
Transition metal complexes have interesting applications in photocatalysis and solar energy conversion. Time-resolved X-ray methods present exciting opportunities to investigate the photochemical mechanisms at play in such reactions. The BCPC group has been developing several such methods. In particular, we have been pioneering multi-modal ultrafast X-ray studies demonstrating the power of combining hard X-ray scattering and spectroscopy to directly quantify the initial coupling of electronic and structural configurations with atomic resolution and spin-state specificity[1]. Various members of the group have active research interests in this area. T. van Driel is interested in studying chemical dynamics with a focus on X-ray solution phase scattering (XSS)[2] combined with x-ray spectroscopy[3]. L. Gee is currently interested in utilizing ultrafast X-ray spectroscopy to probe the photochemistry of iron-nitrosyl complexes and their applications to human health. The group works in close collaboration with SSRL scientists (D. Sokaras group) to promote this area of science and take advantage of the synergies between the two SLAC light sources and, in particular, to exploit the complementarity with the SSRL beamline 15 dedicated to ps time-resolved spectroscopy. More detailed examples in this area can be found here.
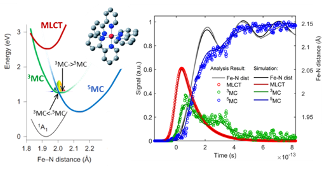
Another focus of the group is the establishment of time-resolved soft X-ray absorption spectroscopy and resonant inelastic X-ray scattering. These techniques will be used to probe valence electronic structure and low-energy electronic excited states at the new chemRIXS instrument within the NEH2.2 endstation. In particular, K. Kunnus is interested in photoinduced dynamics in transition metal coordination complexes utilizing femtosecond time-resolved X-ray spectroscopy methods to track electron transfer and spin transition processes[4]. More examples of the activity of the group in this area of research can be found here.
[1] Alonso-Mori et al. “Photon-in Photon-out Hard X-ray Spectroscopy at the Linac Coherent Light Source" J. Synchrotron Radiat. 22, 612 (2015)
[2] van Driel et al. "Atomistic characterization of the active-site solvation dynamics of a model photocatalyst" Nat. Commun. 7, 13678 (2016)
[3] Kjær et al. "Finding intersections between electronic excited state potential energy surfaces with simultaneous ultrafast X-ray scattering and spectroscopy" Chem. Sci 10, 5749 (2019)
[4]. Kunnus et al., “Quantifying the Steric Effect on Metal–Ligand Bonding in Fe Carbene Photosensitizers with Fe 2p3d Resonant Inelastic X-ray Scattering" Inorg. Chem. 61, 4 (2022)
Biochemistry, coupling atomic and electronic structure of metalloproteins
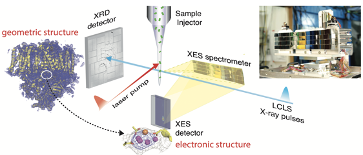
Metalloproteins, accounting for nearly half of all known proteins, are responsible for some of the most difficult and important functions in biology. These functions often involve very subtle structural distortions coupled with changes in electronic structure (e.g. oxidation or spin state). Time-resolved methods based on XFELs are powerful tools for understanding enzyme function and related biochemical processes in operating environments. In addition to accessing fundamental dynamics, ultrafast X-ray pulses can probe the molecular and electronic structure before the onset of X-ray induced redox reactions and related damage mechanisms that are a significant limitation for synchrotron studies at room temperature ("Diffraction before destruction").
The BCPC develops methods for the simultaneous study of the geometric and electronic structure of metalloproteins with diffraction and spectroscopy. Examples of the impact of this approach are illustrated by LCLS studies of metalloproteins (e.g. Ribonucleotide Reductase[1]) and in particular of PS II where time-resolved XES and XRD provides critical information on the valence state of the Mn atoms correlated with changes in atomic distance in the catalytic cluster through the reaction cycle[2]. XES is also an essential control to track the intactness of the proteins during the XRD measurements.
Hard X-ray spectroscopy methods have been developed and extensively used by this group for the study of biochemical systems. One example is the use of time-resolved Fe XES/XAS to follow photoexcitation of cytochrome c, a heme-containing enzyme that serves as an electron transfer protein in biological processes[3]. Another example is the extensive studies of the photochemical dynamics of Vitamin B12 cobalamins, involved in methyl transfer and radical rearrangement, by ultrafast polarized Co K-edge XAS[4].
[1] Srinivas et al., “High-Resolution XFEL Structure of the Soluble Methane onooxygenase Hydroxylase Complex with its Regulatory Component at Ambient Pressure in Two Oxidation States”. JACS 142, 33 (2020)
[2] Kern et al. "Structures of the intermediates of Kok’s photosynthetic water oxidation clock". Nature 563, 421 (2018)
[3] Mara et al. “Metalloprotein entatic control of ligand-metal bonds quantified by ultrafast x-ray spectroscopy”. Science 356, 1276 (2017)
[4] Miller et al. “Polarized XANES Monitors Femtosecond Structural Evolution of Photoexcited Vitamin B12”. JACS, 139, 1894 (2017)
Shock-driven geochemistry under extreme conditions
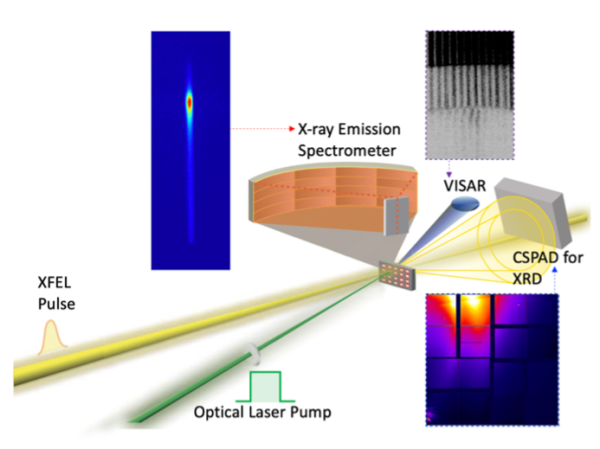
A similar multimodal approach as the one described above to follow geometric and electronic structural changes can be used to study geochemical systems at LCLS. R. Alonso-Mori is driving a scientific program using these methods in laser driven shock compression experiments at the Matter in Extreme Conditions (MEC) instrument. He recently led a study to follow the chemical transformations occurring on a common Earth crust mineral (plagioclase) after the laser-shock induced conditions mimicking those of a large meteoritic impact[1]. More recently the team used a similar approach to study silicates with relevant composition to the Earth mantle. The systems were shock-compressed to induce pressure and temperature conditions similar to those found in the interior of the Earth causing phase transitions coupled with spin changes on the Fe sites[2,3].
[1] Gleason et al., “Ultrafast structural response of shock-compressed plagioclase”. Meteorit. Planet. Sci. 57, 635 (2022)
[2] Morard et al., “In situ X-ray diffraction of silicate iquids and glasses under dynamic and static compression to megabar pressures”. PNAS 117, 11981 (2020)
[3] Shim et al., “Ultrafast X-ray Detection of Low Spin Iron in Molten Silicate at Deep Planetary Interior Conditions”. Submitted
Development of state of the art methods and instrumentation
Pushing the boundaries of what is experimentally feasible at XFELs remains a driving force for the BCPC group. We have been developing X-ray methods and instrumentation tailored for XFELs for over a decade. This includes dispersive spectrometers optimized for our LCLS instruments and multimodal spectroscopy (XES, XAS, EXAFS) and scattering methods (XSS and XRD) to study chemical and biological systems [1,2]. These efforts led to the establishment of the liquid jet standard configurations for XSS/XES/XAS offered to users and being extensively utilized in every run at the XCS and XPP instruments. More details can be found here.
Currently the group’s efforts are devoted to the optimization of the existing methods and to various R&D projects aiming to provide new capabilities. J. Lim is developing a new XAS spectrometer based on polycapillary optics that will enable the measurement of sub mM samples. Significant efforts are being devoted to further develop EXAFS techniques. J. Ormstrup is working towards enabling electrochemistry solutions in the hard X-ray regime. K. Kunnus is developing time-resolved soft X-ray XAS and RIXS methods for the new high repetition rate chemRIXS instrument. F. Fuller’s research is focused on developing non linear X-ray spectroscopies using the unique properties of the XFELs. To that end he has developed a technique called “stochastic spectroscopy”[3] which can be applied to simultaneously measuring scattering and XAS to examine double resonances in non-linear X-ray experiments. Sample delivery methods continue to be developed in conjunction with the SED department with an emphasis on optimizing sample consumption (B. Hayes), the injection of liquid jets in vacuum (D.J Hoffman) and drop on tape systems[4] (F. Fuller). L. Gee is also working on technical developments to extend spectroscopic capabilities at the LCLS towards biomedical applications in the framework of the NIH P41 resource.
A strong focus of the group is devoted to develope spectroscopic capabilities for the currently elusive tender X-ray regime (2-5 keV) at the upcoming TXI instrument. We are developing a novel tender X-ray spectroscopy endstation (TSE)[5] exploiting the high repetition rate of LCLS-II. TSE will enable studies of excited-state electronic structure for a range of atomic edges hard to access anywhere else, including the L-edges of 4d transition metals (Mo, Ru, Ag, etc.) and the K-edges of lighter elements (P, S, Cl, etc.) which form the basis for many biological enzymes and photocatalysts. These experimental capabilities can be applied to a wide range of biological, molecular, and solid-state systems with open scientific questions regarding the excited-state nuclear and electronic structure.
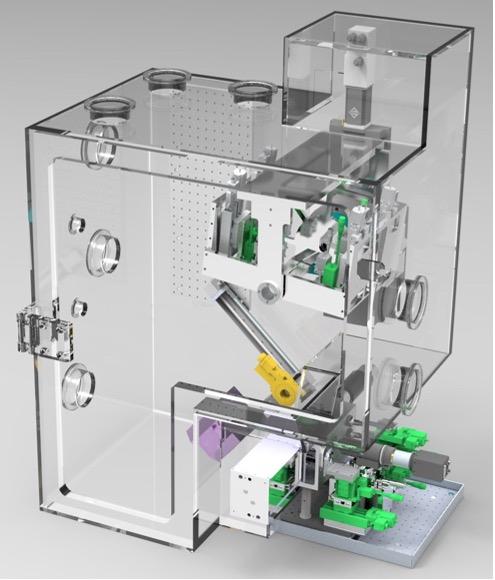
Further developments in preparation for the high repetition rates of LCLS-II and the future LCLS-II HE (1 MHz) will allow us to extend and fully exploit these methods for more challenging systems. Efforts include the development of high sensitivity but photon hungry resonant spectroscopic techniques (e.g. HERFD, RIXS, EXAFS) for very dilute (≲mM) solutions, characteristic of more relevant catalyzers and biochemical complexes in operating conditions.
[1] Alonso-Mori et al., “Energy-dispersive X-ray emission spectroscopy using an X-ray free-electron laser in a shot-by-shot mode”. PNAS, 109, 47 (2012)
[2] van Driel., et al., "Atomistic characterization of the active-site solvation dynamics of a model photocatalyst". Nat. Commun. 7, 1 (2016)
[3] Fuller et al., "Resonant X-ray emission spectroscopy from broadband stochastic pulses at an X-ray free electron laser." Commun. Chem. 4, 1 (2021)
[4] Fuller et al., "Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers." Nat. Methods 14, 443 ( 2017
[5] Abraham et al., “A high-throughput energy-dispersive tender X-ray spectrometer for shot-to-shot sulfur measurements”. J. Synchrotron Radiat. 26, 3 (2019)
Small molecule serial femtosecond crystallography
Small-molecule serial femtosecond crystallography (smSFX) has become a proven method for high quality de novo atomic structure determination from sub-optimal small-molecule microcrystalline powders. smSFX requires no prior crystallographic information about the samples, this capability is particularly useful for material classes that notoriously produce small or defective crystals and possess complex and unpredictable structures. By using the higher photon
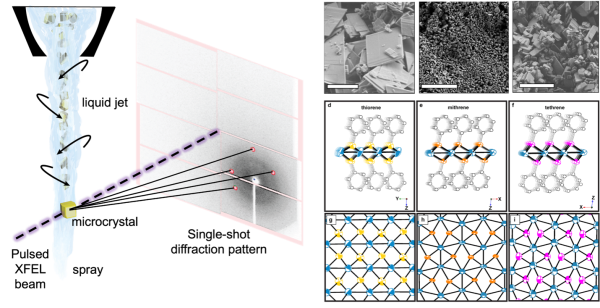
energies available at LCLS, smSFX overall dataset resolution sits at 0.8 Å, making smSFX derived structures comparable to those from single-crystal diffraction at synchrotrons. Many of the structures regularly solved during smSFX experiments belong to low-symmetry triclinic and monoclinic crystal systems frequently seen in other material classes, making smSFX a generally usable technique for chemistry and materials science.
[1] Femtosecond X-Ray Diffraction. Nature 2022, 601 (7893), 360–365. https://doi.org/10.1038/s41586-021-04218-3.
[2] Aleksich, M.; Paley, D. W.; Schriber, E. A., et al. XFEL Microcrystallography of Self-Assembling Silver n-Alkanethiolates. J. Am. Chem. Soc. 2023, 145 (31), 17042–17055. https://doi.org/10.1021/jacs.3c02183.
[3] Kotei, P. A., et al. Engineering Supramolecular Hybrid Architectures with Directional Organofluorine Bonds. Small Sci. 2024, 4 (1), 2300110. https://doi.org/10.1002/smsc.202300110.
